Search
- Page Path
- HOME > Search
- Adrenal Gland
- A Novel Missense PRKAR1A Variant Causes Carney Complex
- Boram Kim, Han Na Jang, Kyung Shil Chae, Ho Seop Shin, Yong Hwy Kim, Su Jin Kim, Moon-Woo Seong, Jung Hee Kim
- Endocrinol Metab. 2022;37(5):810-815. Published online October 4, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1544

- 1,840 View
- 153 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - The Carney complex (CNC) is an autosomal dominant disorder characterized by endocrine and nonendocrine tumors. Loss-of-function variants of protein kinase A regulatory subunit 1 alpha (PRKAR1A) are common causes of CNC. Here, we present the case of a patient with CNC with a novel PRKAR1A missense variant. A 21-year-old woman was diagnosed with CNC secondary to acromegaly and adrenal Cushing syndrome. Genetic analysis revealed a novel missense heterozygous variant of PRKAR1A (c.176A>T). Her relatives, suspected of having CNC, also carried the same variant. RNA analysis revealed that this variant led to nonsense-mediated mRNA decay. In vitro functional analysis of the variant confirmed its role in increasing protein kinase A activity and cyclic adenosine monophosphate levels. This study broadens our understanding of the genetic spectrum of CNC. We suggest that PRKAR1A genetic testing and counseling be recommended for patients with CNC and their families.
-
Citations
Citations to this article as recorded by- Carney complex: A clinicopathologic study on a single family from several Canadian provinces
Alexandra Lao, Julio Silva, Brian Chiu, Consolato M. Sergi
Cardiovascular Pathology.2024; 69: 107599. CrossRef
- Carney complex: A clinicopathologic study on a single family from several Canadian provinces

- Adrenal Gland
- Outcome-Based Decision-Making Algorithm for Treating Patients with Primary Aldosteronism
- Jung Hee Kim, Chang Ho Ahn, Su Jin Kim, Kyu Eun Lee, Jong Woo Kim, Hyun-Ki Yoon, Yu-Mi Lee, Tae-Yon Sung, Sang Wan Kim, Chan Soo Shin, Jung-Min Koh, Seung Hun Lee
- Endocrinol Metab. 2022;37(2):369-382. Published online April 14, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1391
- 3,767 View
- 159 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
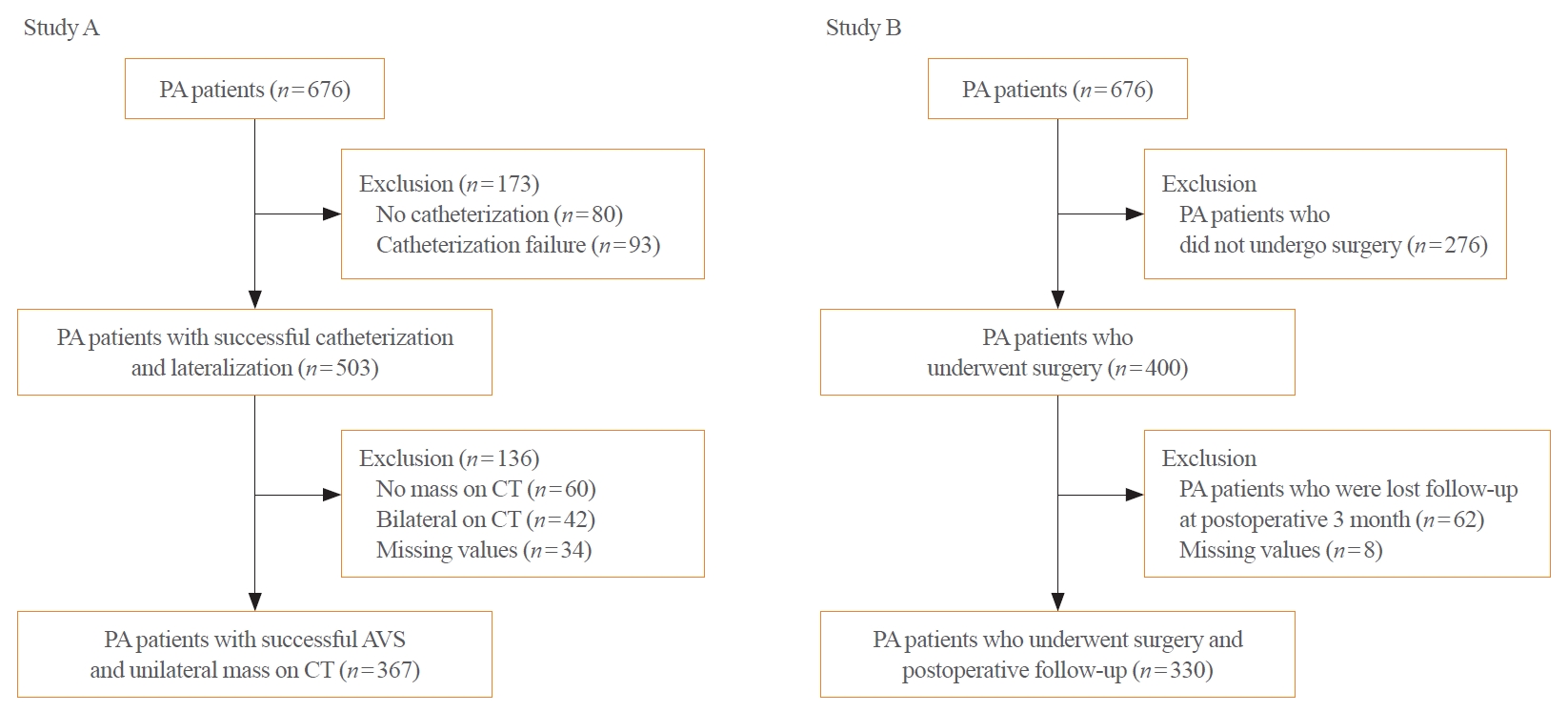
Optimal management of primary aldosteronism (PA) is crucial due to the increased risk of cardiovascular and cerebrovascular diseases. Adrenal venous sampling (AVS) is the gold standard method for determining subtype but is technically challenging and invasive. Some PA patients do not benefit clinically from surgery. We sought to develop an algorithm to improve decision- making before engaging in AVS and surgery in clinical practice.
Methods
We conducted the ongoing Korean Primary Aldosteronism Study at two tertiary centers. Study A involved PA patients with successful catheterization and a unilateral nodule on computed tomography and aimed to predict unilateral aldosterone-producing adenoma (n=367). Study B involved similar patients who underwent adrenalectomy and aimed to predict postoperative outcome (n=330). In study A, we implemented important feature selection using the least absolute shrinkage and selection operator regression.
Results
We developed a unilateral PA prediction model using logistic regression analysis: lowest serum potassium level ≤3.4 mEq/L, aldosterone-to-renin ratio ≥150, plasma aldosterone concentration ≥30 ng/mL, and body mass index <25 kg/m2 (area under the curve, 0.819; 95% confidence interval, 0.774 to 0.865; sensitivity, 97.6%; specificity, 25.5%). In study B, we identified female, hypertension duration <5 years, anti-hypertension medication <2.5 daily defined dose, and the absence of coronary artery disease as predictors of clinical success, using stepwise logistic regression models (sensitivity, 94.2%; specificity, 49.3%). We validated our algorithm in the independent validation dataset (n=53).
Conclusion
We propose this new outcome-driven diagnostic algorithm, simultaneously considering unilateral aldosterone excess and clinical surgical benefits in PA patients. -
Citations
Citations to this article as recorded by- Subtype-specific Body Composition and Metabolic Risk in Patients With Primary Aldosteronism
Seung Shin Park, Chang Ho Ahn, Sang Wan Kim, Ji Won Yoon, Jung Hee Kim
The Journal of Clinical Endocrinology & Metabolism.2024; 109(2): e788. CrossRef - Prognostic models to predict complete resolution of hypertension after adrenalectomy in primary aldosteronism: A systematic review and meta‐analysis
Luigi Marzano, Amir Kazory, Faeq Husain‐Syed, Claudio Ronco
Clinical Endocrinology.2023; 99(1): 17. CrossRef - 2023 Korean Endocrine Society Consensus Guidelines for the Diagnosis and Management of Primary Aldosteronism
Jeonghoon Ha, Jung Hwan Park, Kyoung Jin Kim, Jung Hee Kim, Kyong Yeun Jung, Jeongmin Lee, Jong Han Choi, Seung Hun Lee, Namki Hong, Jung Soo Lim, Byung Kwan Park, Jung-Han Kim, Kyeong Cheon Jung, Jooyoung Cho, Mi-kyung Kim, Choon Hee Chung
Endocrinology and Metabolism.2023; 38(6): 597. CrossRef - Correlation of Histopathologic Subtypes of Primary Aldosteronism with Clinical Phenotypes and Postsurgical Outcomes
Chang Ho Ahn, You-Bin Lee, Jae Hyeon Kim, Young Lyun Oh, Jung Hee Kim, Kyeong Cheon Jung
The Journal of Clinical Endocrinology & Metabolism.2023;[Epub] CrossRef
- Subtype-specific Body Composition and Metabolic Risk in Patients With Primary Aldosteronism

- Clinical Study
- Whole Exome Sequencing Identifies Novel Genetic Alterations in Patients with Pheochromocytoma/Paraganglioma
- Soo Hyun Seo, Jung Hee Kim, Man Jin Kim, Sung Im Cho, Su Jin Kim, Hyein Kang, Chan Soo Shin, Sung Sup Park, Kyu Eun Lee, Moon-Woo Seong
- Endocrinol Metab. 2020;35(4):909-917. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.756
- 5,256 View
- 156 Download
- 10 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Pheochromocytoma and paragangliomas (PPGL) are known as tumors with the highest level of heritability, approximately 30% of all cases. Clinical practice guidelines of PPGL recommend genetic testing for germline variants in all patients. In this study, we used whole exome sequencing to identify novel causative variants associated with PPGL to improve the detection of rare genetic variants in our cohort.
Methods
Thirty-six tested negative for pathogenic variants in previous Sanger sequencing or targeted gene panel testing for PPGL underwent whole exome sequencing. Whole exome sequencing was performed using DNA samples enriched using TruSeq Custom Enrichment Kit and sequenced with MiSeq (Illumina Inc.). Sequencing alignment and variant calling were performed using SAMtools.
Results
Among previously mutation undetected 36 patients, two likely pathogenic variants and 13 variants of uncertain significance (VUS) were detected in 32 pheochromocytoma-related genes. SDHA c.778G>A (p.Gly260Arg) was detected in a patient with head and neck paraganglioma, and KIF1B c.2787-2A>C in a patient with a bladder paraganglioma. Additionally, a likely pathogenic variant in BRCA2, VUS in TP53, and VUS in NFU1 were detected.
Conclusion
Exome sequencing further identified genetic alterations by 5.6% in previously mutation undetected patients in PPGL. Implementation of targeted gene sequencing consisted of extended genes of PPGL in routine clinical screening can support the level of comprehensive patient assessment. -
Citations
Citations to this article as recorded by- Patient Sex and Origin Influence Distribution of Driver Genes and Clinical Presentation of Paraganglioma
Susan Richter, Nicole Bechmann
Journal of the Endocrine Society.2024;[Epub] CrossRef - Case Report: Aggressive neural crest tumors in a child with familial von Hippel Lindau syndrome associated with a germline VHL mutation (c.414A>G) and a novel KIF1B gene mutation
Lucie Landen, Anne De Leener, Manon Le Roux, Bénédicte Brichard, Selda Aydin, Dominique Maiter, Philippe A. Lysy
Frontiers in Endocrinology.2023;[Epub] CrossRef - A Case of Pheochromocytoma as a Subsequent Neoplasm in a Survivor of Childhood Embryonal Rhabdomyosarcoma
Rozalyn L. Rodwin, Sanyukta K. Janardan, Erin W. Hofstatter, Nina S. Kadan-Lottick
Journal of Pediatric Hematology/Oncology.2022; 44(2): e585. CrossRef - Mutations of 1p genes do not consistently abrogate tumor suppressor functions in 1p-intact neuroblastoma
Chik Hong Kuick, Jia Ying Tan, Deborah Jasmine, Tohari Sumanty, Alvin Y. J. Ng, Byrrappa Venkatesh, Huiyi Chen, Eva Loh, Sudhanshi Jain, Wan Yi Seow, Eileen H. Q. Ng, Derrick W. Q. Lian, Shui Yen Soh, Kenneth T. E. Chang, Zhi Xiong Chen, Amos H. P. Loh
BMC Cancer.2022;[Epub] CrossRef - A case of juvenile-onset pheochromocytoma with KIF1B p.V1529M germline mutation
Masahiro Nezu, Yosuke Hirotsu, Kenji Amemiya, Miho Katsumata, Tomomi Watanabe, Soichi Takizawa, Masaharu Inoue, Hitoshi Mochizuki, Kyoko Hosaka, Toshio Oyama, Masao Omata
Endocrine Journal.2022; 69(6): 705. CrossRef - Systematic analysis of expression profiles and prognostic significance for MMDS-related iron–sulfur proteins in renal clear cell carcinoma
Ling Yang, Yu-Xin Chen, Ying-Ying Li, Xiao-Juan Liu, Yong-Mei Jiang, Jia Mai
Scientific Reports.2022;[Epub] CrossRef - Diagnosis for Pheochromocytoma and Paraganglioma: A Joint Position Statement of the Korean Pheochromocytoma and Paraganglioma Task Force
Eu Jeong Ku, Kyoung Jin Kim, Jung Hee Kim, Mi Kyung Kim, Chang Ho Ahn, Kyung Ae Lee, Seung Hun Lee, You-Bin Lee, Kyeong Hye Park, Yun Mi Choi, Namki Hong, A Ram Hong, Sang-Wook Kang, Byung Kwan Park, Moon-Woo Seong, Myungshin Kim, Kyeong Cheon Jung, Chan
Endocrinology and Metabolism.2021; 36(2): 322. CrossRef - Recurrent Germline Mutations of CHEK2 as a New Susceptibility Gene in Patients with Pheochromocytomas and Paragangliomas
Yinjie Gao, Chao Ling, Xiaosen Ma, Huiping Wang, Yunying Cui, Min Nie, Anli Tong, Dario De Biase
International Journal of Endocrinology.2021; 2021: 1. CrossRef
- Patient Sex and Origin Influence Distribution of Driver Genes and Clinical Presentation of Paraganglioma

- Obesity and Metabolism
- Vav3, a GEF for RhoA, Plays a Critical Role under High Glucose Conditions
- Jie Sha, Jungsik Na, Jung Ok Lee, Nami Kim, Soo Kyung Lee, Ji Hae Kim, Ji Wook Moon, Su Jin Kim, Hye Jeong Lee, Jong-Il Choi, Sun Hwa Park, Hyeon Soo Kim
- Endocrinol Metab. 2014;29(3):363-370. Published online September 25, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.3.363
- 3,729 View
- 39 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The role of small GTPase molecules is poorly understood under high glucose conditions.
Methods We analyzed the expression pattern of Vav3 in skeletal muscle C2C12 cells under high glucose culture condition with reverse transcription-polymerase chain reaction and Western blot analysis. We also measured glucose uptake using isotope-labelled glucose.
Results We showed that expression of Vav3 (a guanine nucleotide exchange factor for RhoA) increased. mRNA and protein levels in skeletal muscle C2C12 cells under high glucose conditions. The AMP-activated protein kinase (AMPK) activator AMPK agonist 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside (AICAR) suppressed high glucose-induced Vav3 induction. In addition, exposure of cells to high glucose concentration increased the phosphorylation of PAK-1, a molecule downstream of RhoA. The phosphorylation of paxillin, a downstream molecule of PAK-1, was also increased by exposure to high glucose. Phosphorylation of these molecules was not observed in the presence of AICAR, indicating that AMPK is involved in the RhoA signal pathway under high glucose conditions. Knock down of Vav3 enhances metformin-mediated glucose uptake. Inhibition of AMPK blocked the increases of Vav3 knock down-induced glucose uptake. Metformin-mediated Glut4 translocation was also increased by Vav3 knock-down, suggesting that Vav3 is involved in metformin-mediated glucose uptake.
Conclusion These results demonstrate that Vav3 is involved in the process of metformin-mediated glucose regulation.
-
Citations
Citations to this article as recorded by- Peripheral origin exosomal microRNAs aggravate glymphatic system dysfunction in diabetic cognitive impairment
Lin Zhang, Dongna Li, Pengrong Yi, Jiangwei Shi, Mengqing Guo, Qingsheng Yin, Dingbin Liu, Pengwei Zhuang, Yanjun Zhang
Acta Pharmaceutica Sinica B.2023; 13(7): 2817. CrossRef - A current overview of RhoA, RhoB, and RhoC functions in vascular biology and pathology
Robert Eckenstaler, Michael Hauke, Ralf A. Benndorf
Biochemical Pharmacology.2022; 206: 115321. CrossRef - Rho Family GTPases and Rho GEFs in Glucose Homeostasis
Polly A. Machin, Elpida Tsonou, David C. Hornigold, Heidi C. E. Welch
Cells.2021; 10(4): 915. CrossRef - Pharmacological Modulators of Small GTPases of Rho Family in Neurodegenerative Diseases
William Guiler, Addison Koehler, Christi Boykin, Qun Lu
Frontiers in Cellular Neuroscience.2021;[Epub] CrossRef - Association of VAV2 and VAV3 polymorphisms with cardiovascular risk factors
Nuria Perretta-Tejedor, Javier Fernández-Mateos, Luis García-Ortiz, Manuel A. Gómez-Marcos, José I. Recio-Rodríguez, Cristina Agudo-Conde, Emiliano Rodriguez-Sánchez, Ana I. Morales, Francisco J. López-Hernández, José M. López-Novoa, Rogelio González-Sarm
Scientific Reports.2017;[Epub] CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Peripheral origin exosomal microRNAs aggravate glymphatic system dysfunction in diabetic cognitive impairment


 KES
KES

 First
First Prev
Prev



